|
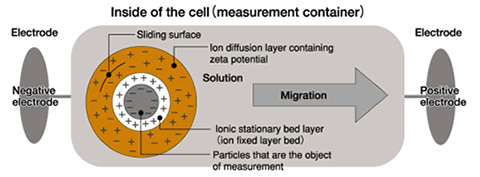
What is zeta potential?
When a voltage
is applied to a solution in which colloidal particles are dispersed,
particles are attracted to the electrode of the opposite polarity.
The actual particle migrates to the electrode as a part of the
system which includes the charged particle, and layers of solvent
and ions that are bound with varying degrees of strength to the
particle. At a certain distance from the surface of the
particle there is a layer of ions that are mobile and are removed as
the particle system moves through the solvent as a result of the
electrostatic attraction to the electrode. This layer is
called the “sliding surface.”
Zeta potential is considered to be the electric potential of this
inner area including this conceptual "sliding surface".
As this electric potential approaches zero, particles tend to
aggregate due to Van der Waals force overcoming electrostatic
repulsion. Higher zeta potentials will cause colloidal systems to
remain stable and dispersed.


Dispersed particles
|

Aggregated particles |
As this electric potential
approaches zero, particles tend to aggregate. Higher zeta potentials will
cause particles to repel due to like charges.
The static layer is computed using the following formula (which is embedded
in the Zeecom software).
The static layer is computed using the following formula (which is
embedded in the Zeecom software).
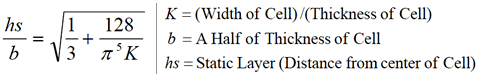
Zeta Potential (Smoluchowski’s Formula)
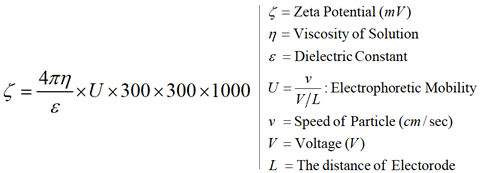
The Zeecom unit uses a microscope to observe particle electrophoresis
migration on a monitor while performing image processing to determine the
particle migration speed, which is then converted into the zeta potential value.
To eliminate errors due to electroosmotic flow caused by any electrostatic
charge on the measurement cell, the zeta potential is measured at the static
layer, whose position can be calculated from the width and depth of the cell (the software performs this calculation automatically).
Click
HERE for a demo of the software
|